Sensational Tips About How To Tell If A Precipitate Forms

A lot of ionic compounds dissolve in water, dissociating into individual ions.
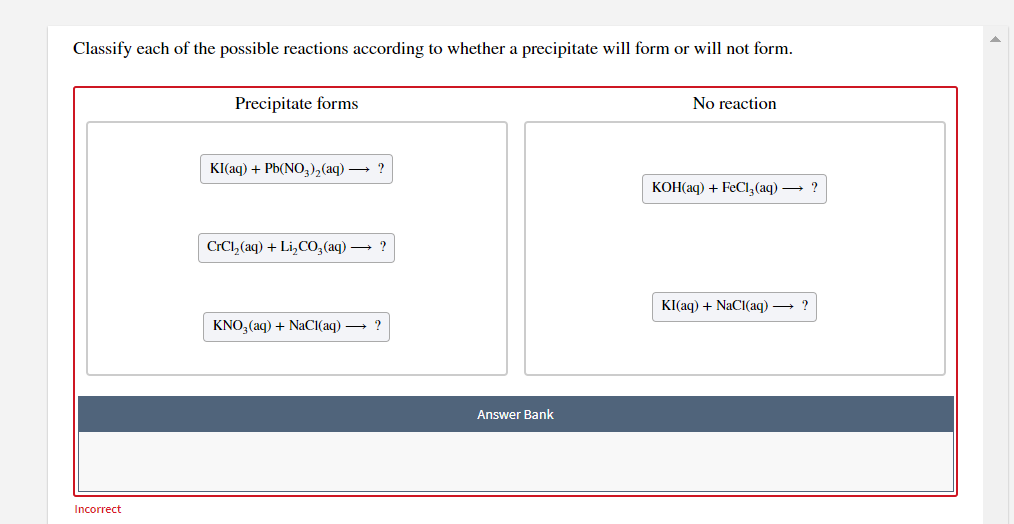
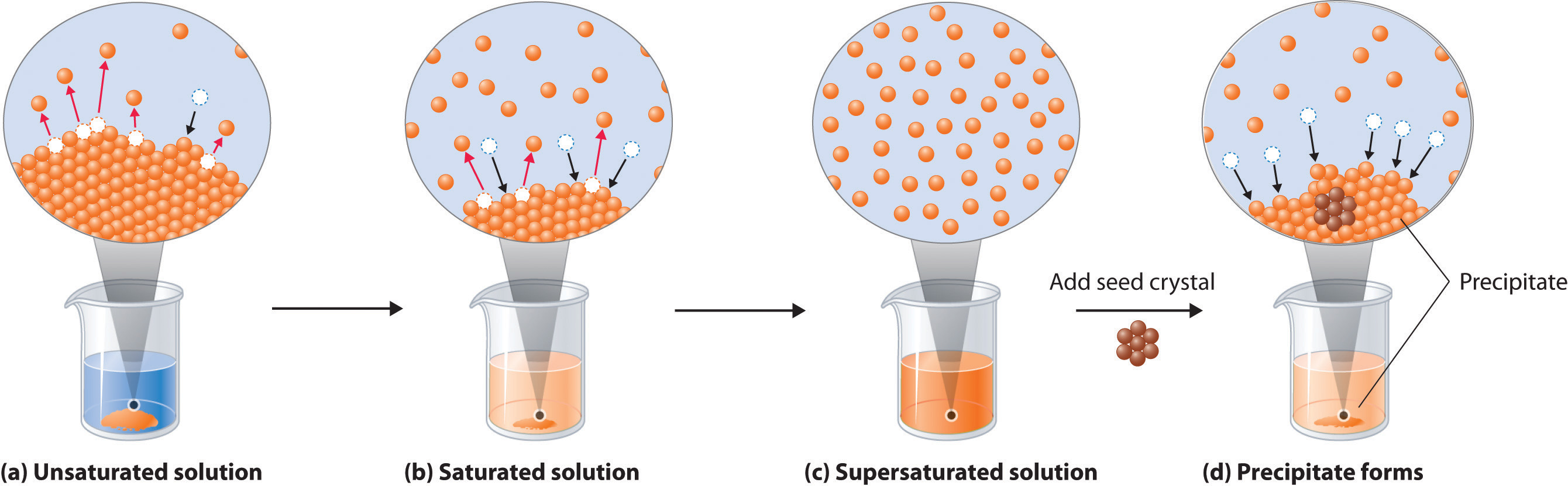
How to tell if a precipitate forms. When writing a chemical reaction, the presence of a precipitate may be indicated by following the chemical formula with an arrow pointing downward: This occurs specifically when two aqueous solutions (typically clear solutions). Identify the reactants and products.
What is the precipitate in the following aqueous reaction? Two aqueous solutions can mix and the ions inside can. It says that ai systems that can be used in different applications are.
Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Der kreole/wikimedia commons) example 1. Read through the given information in the problem for the chemical reaction.
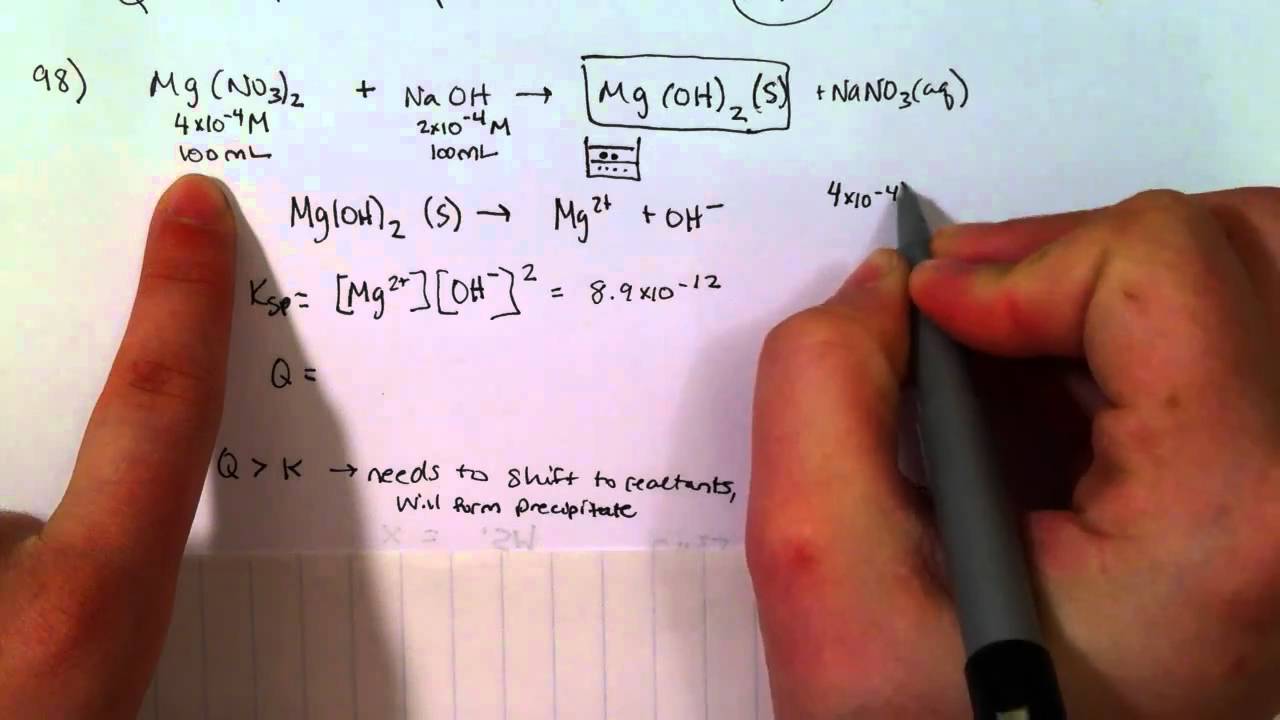
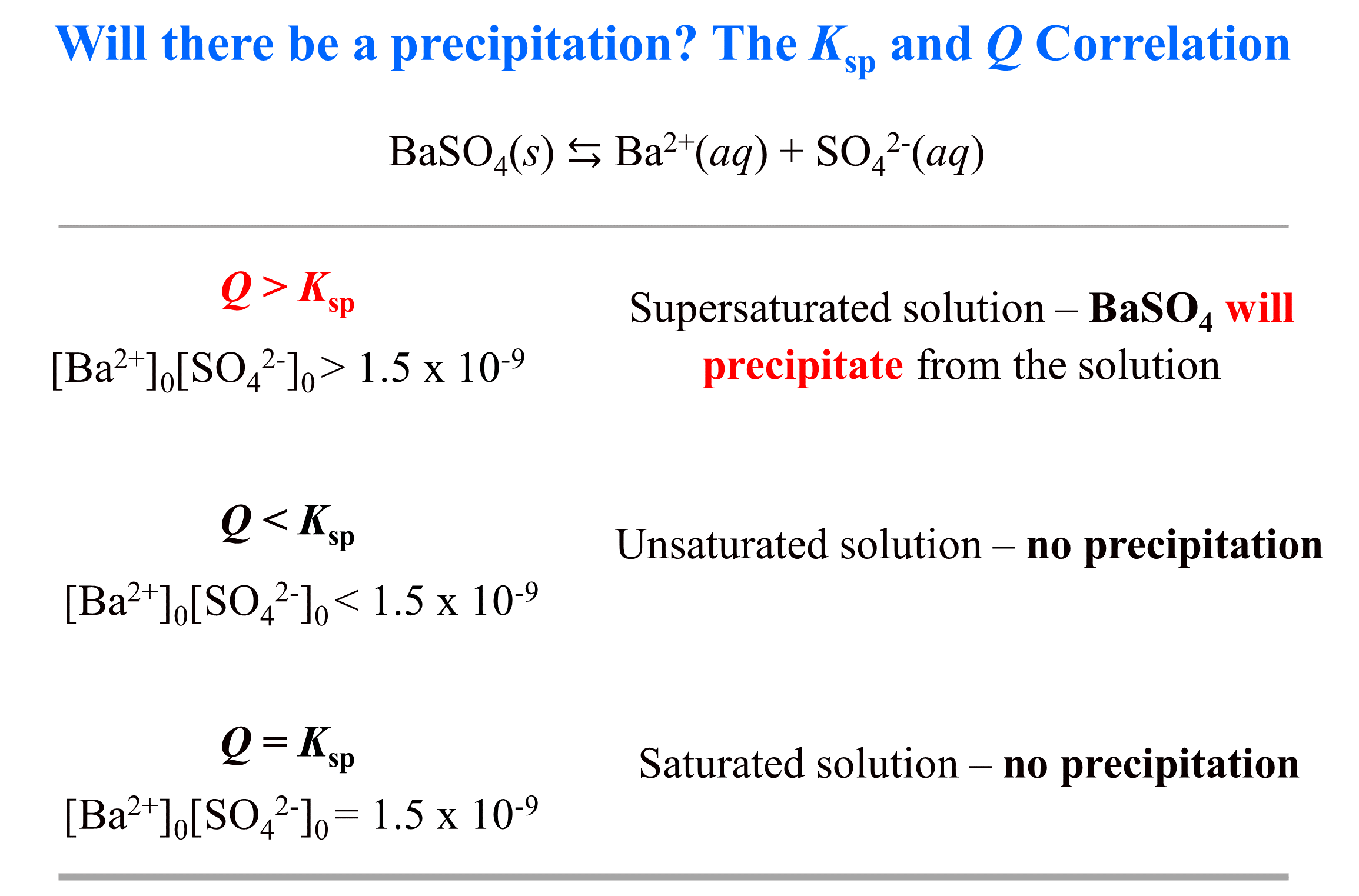
The formation of the precipitate lowers the concentration of each. But when two ions find each other that form an insoluble compound, they. If the value of the ion product is greater than the value of the \(k_\text{sp}\), then a precipitate will form.
Predict the result of mixing. 17k views 7 years ago general chemistry 1. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products.
We described a precipitation reaction in. Agno3 + nacl → agcl + nano3. A precipitate is a solid formed in a double displacement reaction.
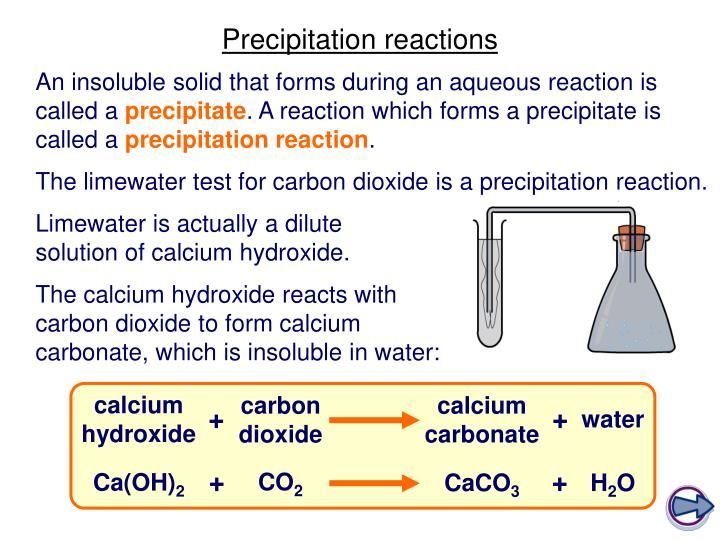
A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. In april 2021, the european commission proposed the first eu regulatory framework for ai. Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.
Solution a because barium chloride and lithium sulfate are strong electrolytes, each dissociates completely. Many reactions of this type involve the exchange of ions between ionic. Chemical reactions involve chemical changes that result in the formation of new compounds under some.
Determining if a precipitate will form in a solution | chemistry with cat a precipitate can form 2 ways: A precipitate of pbi2 forms when solutions containing pb2+ and i− are mixed. When a colorless solution of silver nitrate is.





:max_bytes(150000):strip_icc()/precipitate-589cb8953df78c47581a9014.jpg)